How To Read Patch Clamp
Patch-clamp is the gold standard technique for high-fidelity analysis of the electrical properties and functional connectivity of neurons. Several patch clamp configurations can be used depending on the research interests, but in all cases, electrophysiological recordings are produced using a glass micropipette in contact with a patch of the neuron’s membrane. In the cell-attached mode, the membrane patch is left intact allowing the recording of ion channels within the patch as well as action potentials.
Applying a pore-forming agent, such as amphotericin, in the pipette results in a perforated patch, which establishes electrical continuity whilst preventing the dialysis of intracellular proteins. However, the most commonly used patch-clamp mode is the whole-cell mode where the membrane patch is disrupted by briefly applying strong suction to establish electrical and molecular access to the intracellular space. This has two main configurations: the voltage-clamp mode, in which the voltage is held constant allowing the study of ionic currents, and the current-clamp mode, in which the current is controlled enabling the study of changes in membrane potential. Several books have been written describing this technique in detail. Described here, is a simplified protocol of the whole-cell patch clamp technique, for use in neuronal cultures. This protocol has been used to generate the results described below. The solutions and voltage and current steps used are specific for these recordings and can be modified according to the scientist’s requirements.
Voltage Clamping a Patch. • To use the voltage clamp to demonstrate that a portion of the Na channels are inactivated at rest.
Materials Required Solutions Artificial cerebrospinal fluid (aCSF) Composition: 126 mM NaCl, 3 mM KCl, 2 mM MgSO4, 2 mM CaCl2, 1.25 mM NaH2PO4, 26.4 mM NaHCO3 and 10 mM glucose. Preparation: Make up 1 L 10X stock solution containing only NaHCO3, another 1 L 10X stock solution containing the rest of the components and store for up to one week at 4 °C. Before recording, prepare 1X solution; adjust osmolarity to 290 mOsm (+/- 10 mOsm) and bubble with carbogen (95% O2 – 5% CO2).
IMPORTANT: This aCSF recipe uses a bicarbonate buffer; alternatively, HEPES (10-15 mM) can be used to buffer the pH of the solution. If a HEPES buffered solution is used, NaHCO3 and CO2 bubbling are not required. Intracellular solution Composition: 115 mM K-Gluconate, 4 mM NaCl, 0.3 mM GTP-NaCl, 2 mM ATP-Mg, 40 mM. Preparation: Make up 1X solution; adjust the pH to 7.2 with KOH and the osmolarity at 270 mOsm L-1 (+/- 10 mOsm L-1). Aliquot the solution and store at -20 °C.
Before recording, thaw an aliquot and filter it using a standard 0.2 μm filter. Use a 1 mL syringe with a microloader tip to load the recording pipettes. If analysis of cellular morphology post hoc is required, include an intracellular dye or label in the internal solution (e.g. Biocytin, neurobiotin, etc) and reduce the concentration of K-Gluconate correspondingly. Preparation 1. Plate the neurons a few days prior to recording onto coverslips.
Turn on all the equipment and set the pump to perfuse aCSF through the recording chamber (a commonly used speed for whole-cell patch clamp in cultures is 1.5 mL per minute). IMPORTANT: a perfusion speed over 2 mL per minute might lead to movements of the recording pipette and lifting of the cells from the coverslip. Place the coverslip with cells in the recording chamber with the cells facing up.
Use a glass capillary puller to make two recording pipettes from a borosilicate glass capillary with a resistance of between 3 and 7 MΩ when filled with K-Gluconate based internal solution. IMPORTANT: 5 MΩ is the standard resistance used for most recordings. Lower resistance pipettes (of 3-4 MΩ) will give a lower series resistance and thus, will be better for voltage-clamp recordings. However, the tip of the glass pipette will be larger than for higher resistance pipettes and, as a consequence, the seal formation will be challenging and the seal will be more difficult to keep stable over time. Higher resistance glass pipettes (of 6-7 MΩ, with a thinner tip) are easier to form a seal with and can be more suitable for prolonged current-clamp recordings; however, they are slightly more difficult to break through the membrane into whole-cell mode and will give a higher series resistance, sometimes too high for reliable voltage-clamp recordings.
I.e.SketchUp 7, 8 or 2013. Su podium v2 s. • Please do a simple model test.
Fill a 1 mL syringe with 200 µL of intracellular solution, connect a 0.2 µm pore filter to the syringe and attach a micro-loader tip to the filter. Alternatively, filter 200 µL of intracellular solution with a 0.2 µm pore filter, fill a 1 mL syringe with the filtered solution and attach a micro-loader tip to the syringe barrel. Main procedure 1. Find a cell to patch. Do not move the microscope stage for the rest of the procedure.
If using an upright microscope, move the objective outside the bath. Use the syringe linked to the filter and micro-loader tip to fill halfway a borosilicate pipette with intracellular solution. Tap the pipette a few times to eliminate any air bubbles that might be present in the tip of the pipette.
Place the glass pipette in the pipette holder. Place the pipette tip in the bath and focus the tip. Once the pipette is in the bath, apply very light positive pressure through the pressure control system and hold the pressure in the pipette by closing the three-way valve. Approach the coverslip by moving the micromanipulator and monitoring the pipette height on the screen. Always focus at or below the tip of the glass pipette. Stop moving the glass pipette just before approaching the cell layer on the coverslip, making sure that the cell bodies are not in focus at this stage. Change the settings of the micromanipulator to smooth and slow motion.
Set the amplifier to voltage-clamp and correct the pipette offset so the currents measured at that point are considered as 0 pA. Apply a seal test (a 10 mV test pulse at 100 Hz) through the recording electrode. IMPORTANT: The oscilloscope should show the square current response to the seal test. Following Ohm’s law (current (I)= voltage (E) / resistance(R)), the amplitude of the response will depend on the resistance of the pipette. Approach the cell body moving the glass pipette along its long axis until the tip touches the cell and a very small dimple is seen on the cell’s membrane. IMPORTANT: Do not patch the nucleus of the cell. Approach the cell away from the nucleus.
Release the positive pressure to obtain a GΩ seal. The pressure should be so light that a seal could form spontaneously only by approaching the cell. IMPORTANT: A GΩ seal is characterised by a resistance that reaches at least 1 GΩ. The current response to the seal test should be very small and if the scale of the oscilloscope is kept unchanged, the response should appear almost flat. Once a GΩ seal has been formed, change the voltage clamp to a negative voltage close to the expected cell resting potential (- 60 to -70 mV) and correct for fast capacitance. To break through the membrane, apply light and short suction pulses using a syringe (or by mouth only if it is safe to do so). If the membrane does not break, try applying stronger suction or apply brief electrical pulses through the pipette if the patch clamp amplifier has a ‘zap’ function.
IMPORTANT: Since the membrane of the cell acts as a capacitor, when the glass pipette has gained access to the intracellular space, the current response to the seal test should show an exponential decay. Acquire and analyse recordings using the appropriate software. A) For current clamp experiments, change to current-clamp mode, read the resting membrane potential at zero current, and adjust the membrane potential to -60 to -70 mV by applying current if required. To analyse the passive membrane properties and the spiking properties of the cells, apply alternate steps of negative and positive current.
To confirm the spikes seen are sodium spikes, tetrodotoxin (TTX) can be used to block voltage-dependent sodium channels. B) For voltage-clamp recordings, continue in voltage-clamp mode and remove the seal test.
Hold the cell at -70 mV to record spontaneous excitatory postsynaptic currents (EPSCs) and at 0 mV to record spontaneous inhibitory postsynaptic currents (IPSCs). Blockers of AMPA receptors, NMDA receptors or GABA receptors can be applied to better isolate the currents of interest or to confirm the presence of a specific current. Whole-cell patch clamp can be used to characterize the maturation of neuronal cultures, both at the level of individual cells and at the network’s connectivity level. As neurons derived from AxolNSCs mature over time, the number of cells spiking increased up to 100% of the total number of neurons recorded at one month after plating ( Figure 3A). The maturation stage of neurons is reflected by their spiking profile. Over the course of maturation, neurons express more voltage-dependent Na+ channels causing higher amplitude action potentials and more K+ channels producing a reduction in the spike’s width ( Figure 3B and C). Along with a modification in the spiking profile, synaptic connections start to appear after one month in culture (F igure 3E).
The enrichment on excitatory and inhibitory connections to cells after 45 days in culture suggests a fully mature neuronal network ( Figure 3F). Electrophysiological characterisation of neurons derived from Axol Cortical NSCs.
Patch Clamp
Number of cells recorded that showed evoked action potentials compared to the number of total cells recorded. Three different developmental stages were analysed: 10 to 15 days after plating (DAP) in coverslips, 25 to 30 DAP and 40 to 45 DAP. Representative traces of evoked action potentials.
Developmental profile of the spike properties of neurons derived from NSCs. Voltage clamp recording at -70 mV from NSCs at 10 to 15 DAP. No synaptic currents were detected.
25 to 30 DAP, some synaptic currents were observed. These currents were excitatory postsynaptic currents (EPSCs) and were blocked by CNQX (10 µM), an AMPA and kainate receptor blocker. Fully mature neurons at 40 to 45 days post-plating showed both EPSCs and inhibitory postsynaptic currents (IPSCs), which could be blocked using a GABAA receptor blocker (gabazine, 2 µM). Inhibitory postsynaptic currents (IPSCs) were recorded at 0 mV.
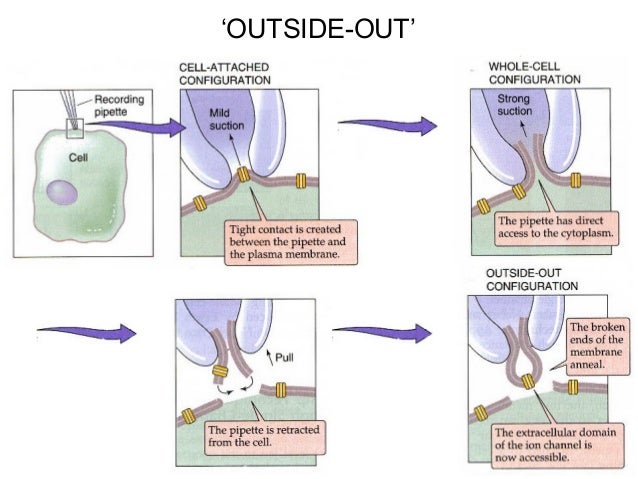
. patch clamp recording;. patch clamp recording, distinct techniques/or configurations;. patch recording configurations;. tight electrical seal, tip of patch pipette and cell membrane;. electrical resistance, calculated using Ohm's law;. cell-attached/whole-cell transition, voltage command pulse;.
cartoon representation, four configurations of patch clamp;. recording from patches of cell membrane;.
sources of error using voltage-clamp, for recording membrane currents;. isolated patches of membrane, and recordings.